Empirical Formula Of Magnesium Chloride
Magnesium Chloride Formula is an important one for students. They should learn and know about information technology. Magnesium chloride is one of the naturally occurring inorganic compounds that has a wide variety of application in industries, medical fields and it is even an important mineral for human being beings.
Magnesium chloride is basically a common salt that is typical ionic halides and they are highly soluble in water. This compound comes in either anhydrous or multiple hydrated crystal forms.
Magnesium Chloride Chemical Formula
During the reaction between magnesium and chlorine, Magnesium has a trend to give up two electrons. Information technology has a charge of +2. Meanwhile, chlorine is willing to have in one electron giving it a charge of -1. However, two chlorine atoms are needed to accept the two electrons from magnesium in lodge to complete it'south octate. When this happens the overall charge becomes nil. Therefore, the chemic formula of magnesium chloride is given as MgCl2.
| Formula | MgClii |
| Molar Mass | 95.211 k/mol |
| Density | 2.32 yard/cm³ |
| Melting Point | 714 °C |
| Humid Point | one,412 °C |
Magnesium Chloride Structural Formula
The structural formula of magnesium chloride is represented equally;
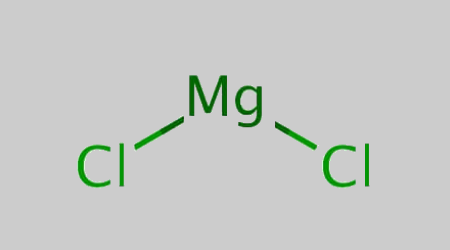
Do stay tuned to BYJU'South to know more about the dissimilar formulas of of import chemical compounds.
Empirical Formula Of Magnesium Chloride,
Source: https://byjus.com/magnesium-chloride-formula/
Posted by: quirogaughtmed.blogspot.com


0 Response to "Empirical Formula Of Magnesium Chloride"
Post a Comment